Introduction: Growth in the number of older cancer survivors in the face of projected healthcare workforce shortages is challenging the US health-care system in delivering appropriate care. A risk-stratified approach based on burden of morbidity and healthcare expertise is urgently needed to provide efficient and cost-effective care. We examined the cumulative, non-malignant, condition-specific hospitalization rates and associated spending in older NHL survivors compared to non-cancer controls, using a population-based approach, to develop evidence for risk-stratified care.
Methods: Using SEER-Medicare data, we identified 14,533 patients diagnosed with NHL at age >65y (2008-2015). An age, race, sex, and follow-up time comparable non-cancer cohort (n=14,533) was also identified. Hospitalizations for 10 health conditions were used to examine cumulative utilization burden of non-malignant morbidity by time from diagnosis to 5y or date of bone marrow transplant (whichever came first), censoring at 6 mo prior to death or end-of-study (12/31/2016). We estimated the average number of hospitalizations per 100 individuals, up to 1y and 5y, accounting for the competing risk of leaving the cohort (BMT or 6 months prior to death). We calculated incident rate ratios [IRR] per person-time comparing NHL patients to controls controlling for pre-cancer comorbidity, Dual Medicaid-Medicare coverage, race/ethnicity, and pre-NHL hospitalization. We calculated Medicare spending per hospitalization and per person-year (adjusted to 2016 pricing), defining high hospitalization or high spending using the 90th percentile cutpoint for predicted hospitalization or spending.
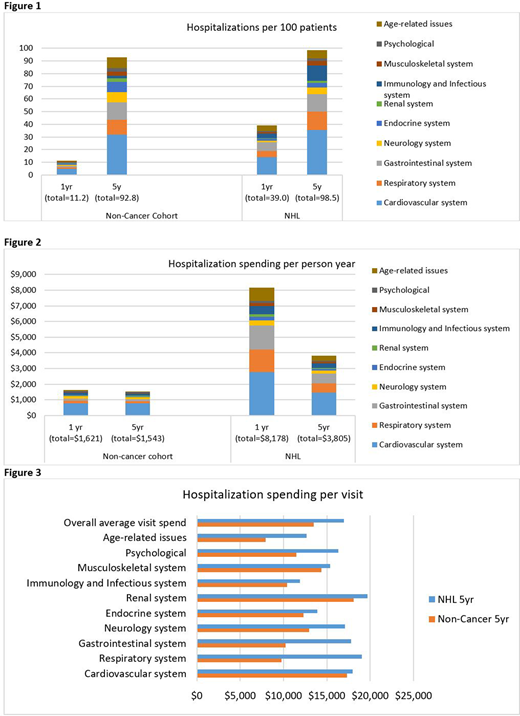
Results:Hospitalization: In Fig 1, we summarize the average number of condition-specific hospitalizations/100 individuals by 1y and by 5y. Adjusting for demographics and pre-existing comorbidity, NHL patients were significantly more likely to be hospitalized when compared with controls (IRR1y=3.08, IRR5y=1.54, all p<0.001). Compared with controls, NHL patients were at higher risk for hospitalizations related to cardiovascular disease (IRR1y=3.08, IRR5y=1.54, all p<0.001), age-related conditions (IRR1y=7.14, IRR5y=2.53, all p<0.001), gastrointestinal (GI) disease (IRR1y=5.84, IRR5y=2.30, all p<0.001), and pulmonary conditions (IRR1y=3.06, IRR5y=1.36, p<0.001). Factors associated with high hospitalization rates included a diagnosis of diffuse large B cell lymphoma (RR=6.36, p<0.001) and ≥2 pre-existing comorbidities (RR=10.1, p<0.001), non-white race/ethnicity (Black: RR=1.74, p<0.001; Hispanic (RR=1.97, p<0.001), residence in low-education area (RR=1.49, p<0.001) or low-income area (RR=37, p<0.001), or rural residence (RR=1.38, p<0.001). The high-hospitalization subgroup (n=1,435) had 3.24 times the hospitalization rate when compared with the lowest-hospitalization subgroup (p<0.001). Spending: Average hospitalization spending per person-year was higher in the NHL cohort (Y1: $8,178 vs. $1,621; 5y: $3,805 vs. $1,543) when compared with non-cancer controls (Fig 2). The average spending per hospitalization over 5y was also higher for the NHL cohort ($16,950 vs. $13,474, Fig 3). Pulmonary conditions were associated with the largest per hospitalization spending differential between NHL and controls ($18k vs. $9k). Other per hospitalization spending disparities between NHL patients and controls included GI disease ($17.8k vs. $10.2k) and age-related conditions ($12.6k vs. $7.9k). The NHL high-spending group was similar to the high-hospitalization group (71% overlap). The highest spending group had 2.5 times the spending compared with the lowest spending (10th percentile) group (p<0.001).
Conclusions: Medicare beneficiaries diagnosed with NHL experience significantly greater rates of non-malignant hospitalizations and spending compared to their non-cancer counterparts. These hospitalizations are related to cardiovascular disease, gastrointestinal disease, pulmonary complications and aging-related conditions. Non-white DLBCL patients from lower SES with ≥2 comorbidities and living in rural areas were at greatest risk of high hospitalization and spending, providing evidence for a targeted risk-stratified care.
Mehta:Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche-Genentech: Research Funding; Merck: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Affimed: Research Funding; Takeda: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Innate Pharmaceuticals: Research Funding; fortyseven Inc/Gilead: Research Funding; Juno Parmaceuticals/BMS: Research Funding; Gelgene/BMS: Research Funding; Oncotartis: Research Funding; Kite/Gilead: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal